Johnson & Johnson, AstraZeneca resume vaccine trials
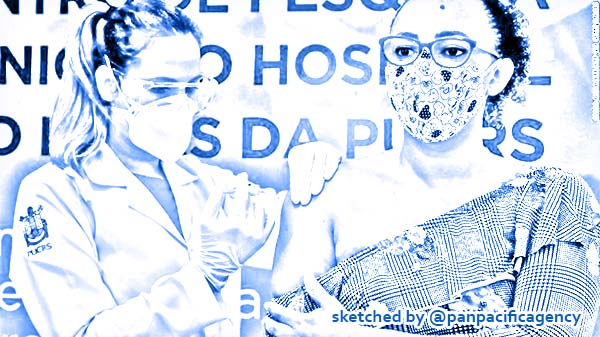
A volunteer receives a COVID-19 vaccine produced by Chinese company Sinovac Biotech at the Sao Lucas Hospital, in Porto Alegre, southern Brazil on August 08, 2020. Photo: CNN. Sketched by the Pan Pacific Agency.
NEW YORK, Oct 24, 2020, Anadolu Agency. Johnson & Johnson and AstraZeneca resumed trials of their experimental COVID-19 vaccine after approval by regulators, local media said on Friday, Anadolu Agency reported.
The US Food and Drug Administration (FDA) gave the green light to both companies to continue their vaccine trials.
The FDA examined the diseases on subjects, which led the studies to pause, and decided that the diseases did not stem from the COVID-19 vaccines.
“The restart of clinical trials across the world is great news as it allows us to continue our efforts to develop this vaccine to help defeat this terrible pandemic,” said Pascal Soriot, the chief executive officer of AstraZeneca.
On Sept. 6, AstraZeneca’s US trials were paused after a serious neurological illness found in a participant.
On Oct. 12, Johnson & Johnson paused its trials after a study participant became ill.
* Writing by Gozde Bayar